New progress in lithium-sulfur battery research: special sulfides do not react with carbonate electrolytes
2022-02-17
New progress in lithium-sulfur battery research: special sulfides do not react with carbonate electrolytes
With the continuous popularity of electric vehicles, scientists are also trying to find more potential and environmentally friendly power batteries. Recently, a team of engineers from Drexel University introduced their new lithium-sulfur battery in the journal Nature Communications Chemistry. Compared with competing products that rely on expensive raw materials (such as cobalt) and are hindered by many problems, this new research breakthrough has greatly advanced the commercialization of lithium-sulfur batteries.
By exploiting the rare chemical phase of sulfur, the researchers were able to prevent damaging chemical reactions in the battery, and it is expected to demonstrate its great performance potential in the future EV power battery market.
Compared with nickel, cobalt, and manganese, one of the great prospects of lithium-sulfur batteries is that the reserves of sulfur are quite abundant, the cost is low enough, and the performance advantages are also more significant (or several times that of today's lithium-ion batteries).
But before they can be put into commercial use, scientists still have to overcome a hurdle -- the formation of so-called polysulfides.
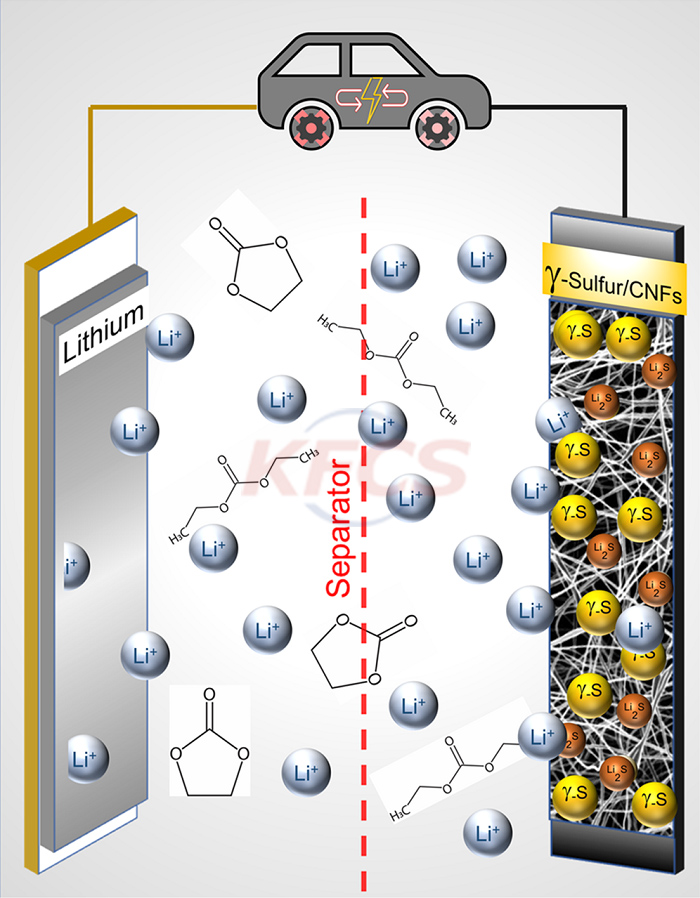
As the battery operates, they enter the electrolyte solution that transports charge back and forth between the cathode and anode, where chemical reactions take place that affect the battery's capacity and lifespan.
Scientists have successfully replaced carbonate electrolytes with ether electrolytes before, and the biggest feature of the latter is that it does not react with polysulfides.
However, this also brings new problems, after all, ether electrolytes are themselves highly volatile and contain low-boiling components - meaning that if heated above room temperature, the battery is likely to fail or melt quickly.
With that in mind, chemical engineers at Drexel University (Drexel.edu) have been working on another set of solutions—starting with designing a new cathode.
It is reported that the new cathode can work with carbonate electrolytes that are currently in commercial use. Made of carbon nanofibers, it has previously been shown to slow the movement of polysulfides in ether electrolytes.
Of course, there is still some work that must be done to verify that the new cathode material works perfectly with carbonate electrolytes.
Lead researcher Vibha Kalra said: "For commercial manufacturers, this is the best way to solve the problem of carbonate electrolyte applications.
Their goal is not to push the industry to adopt an entirely new electrolyte, but to create a new type of cathode that will work in existing lithium-ion electrolyte systems.
The team first tried a technique called steam treatment to trap sulfur in the carbon nanofiber web to prevent dangerous chemical reactions, but unfortunately didn't have the desired effect.
However, it turns out that they formed sulfur crystals in an unexpected way and turned it into a substance called "monoclinic gamma-phase sulfur" (a slight deformation of the element) .
The chemical phase of this sulfide is usually only observed in the high temperature environment of the laboratory or in natural oil wells.
But for scientists, its biggest advantage is that it doesn't react unnecessarily with carbonate electrolytes, eliminating the potential risk of polysulfide formation.
About News
- U.S. to investigate PV modules in four Southeast Asian countries
- The basic principle of all-vanadium redox flow battery
- Industrial chain of power battery recycling
- Vanadium Redox Battery (VRB) technology
- Ultra-high-purity lithium can be recycled at low cost
- Application of vanadium battery in photovoltaic power generation
- Large-scale energy storage: vanadium batteries challenge the lithium battery market
- Vanadium Redox Battery 24V 33ah Rechargeable
- 32kW container type all vanadium flow battery for energy storage
- Vanadium battery market prospect
Products