How Vanadium Flow Redox Batteries Work?
2022-01-18
How Vanadium Flow Redox Batteries Work?
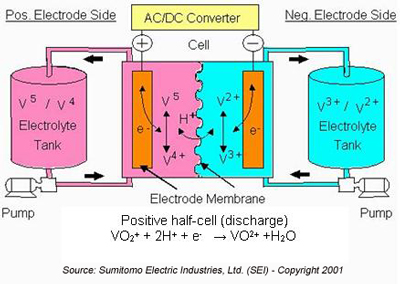
During the discharge cycle, V2+ is oxidized to V3+ in the negative half-cell and an electron is released to do work in the external circuit (either DC or, for AC systems, through an AC/DC converter).
In the positive half-cell, V5+ in the form of VO2+ accepts an electron from the external circuit and is reduced to V4+ in the form of VO2+. Hydrogen (H+) ions are exchanged between the two half-cells to maintain charge neutrality. The hydrogen ions diffuse through the anion or cation-ion permeable polymer membrane that separates the half cells. Charged vanadium species and water can also diffuse across the membrane. The cross-diffusion results in direct energy loss for that cycle. However, when vanadium is the only element present on both sides of the cell, this cross diffusion mechanism does not result in permanent capacity loss, as long as the total vanadium in the system remains constant (i.e., there is no loss due to precipitation).
In a V-only system there is no need to maintain balance between positive and negative sides of the system. In the positive half-cell, the vanadium is present in solution as oxy-cations. These oxy-cations are vulnerable to irreversible precipitation as V2O5 if the electrolyte temperature exceeds ~50-60oC. However, when precipitation occurs, it does so typically in the form of benign compounds, not V2O5.
The normal operating temperature of a VRB is approximately between 10-40oC. Active cooling sub-systems are employed if ambient temperatures exceed 40-45oC. Being able to cool the system actively is an advantage since the system can remain operating without risking any damage to it. By contrast, if integrated cell architectures overheat, the best option is to stop using them until they cool down.
The cell voltage is 1.4-1.6 volts and cell power densities are hundreds mW/cm2 (although Prudent Energy reports their power densities are higher). The DC-DC efficiency of this battery has been reported in the range of 60-80%. According to EPRI, the vanadium redox battery is suitable for power systems in the range of 100 kW to 10 MW, with storage durations in the 2-8 hour range.
The vanadium redox battery offers a relatively high cell voltage, which is favorable for higher power and energy density compared with other true RFBs, like the iron-chromium system. However, the higher voltage and highly oxidative V5+ electrolyte puts more chemical stress on the materials used in the cell electrodes, membranes, and fluid handling components. Cross-transport of vanadium ions across the membrane is also reported as a challenge, and fairly expensive ion-exchange membranes must be used to minimize losses due to cross-membrane transport. These membranes can be vulnerable to fouling, wherein vanadium ions become irreversibly trapped in the membrane and increase resistive losses in the cell. On the other hand, lower cost membranes are under development.
Vanadium is a readily available material, used in steel manufacturing and as a chemical catalyst, which is found naturally and can also be recovered from various waste streams. The market price of vanadium as V2O5 has, however, been fairly volatile since 2017 after enjoying several years of low prices.
About News
- Ultra-high-purity lithium can be recycled at low cost
- Application of all-vanadium redox flow battery
- Volkswagen USA accelerates the layout of power battery recycling
- 500MW! Sweden's OX2 acquires Greek wind and solar projects
- A temperature monitoring device for an all-vanadium redox flow battery case
- Diluted high-concentration electrolyte improves LMB cycling performance, study finds
- Vanadium Redox Battery 24V 33ah Rechargeable
- Significance of power battery recycling
- New South Wales plans to deploy 700MW/1400MWh battery energy storage projects
- The Smart Battery Management System
Products